This collection of pieces is inspired by entropy, the second law of thermodynamics.
There is a finite amount of energy in a closed system. A closed system could be the universe or systems within it like a glass of water or a battery. Where there is low entropy, it broadly means there is a lot of energy in the system and it is spread out unevenly. In a battery for example, the electrons are piled onto one side thus creating an uneven distribution of energy in the system. As the battery power is used, the electrons spread out to a state of eveness, disorder and stagnation which is when the battery goes flat.
This could also be demonstrated by gravity, pulling matter together creating unevenness and therefore high energy and low entropy. The second law of thermodynamics states that entropy is inevitable, meaning the energy will dissipate in a closed system to a stated of evenness, leading to disorder and stagnation, high entropy.
Entropy

40 x 53cm
This is the first piece in the series, demonstrating hot points of energy and that energy dissipating to a state of evenness and disorder.
Systems of Entropy
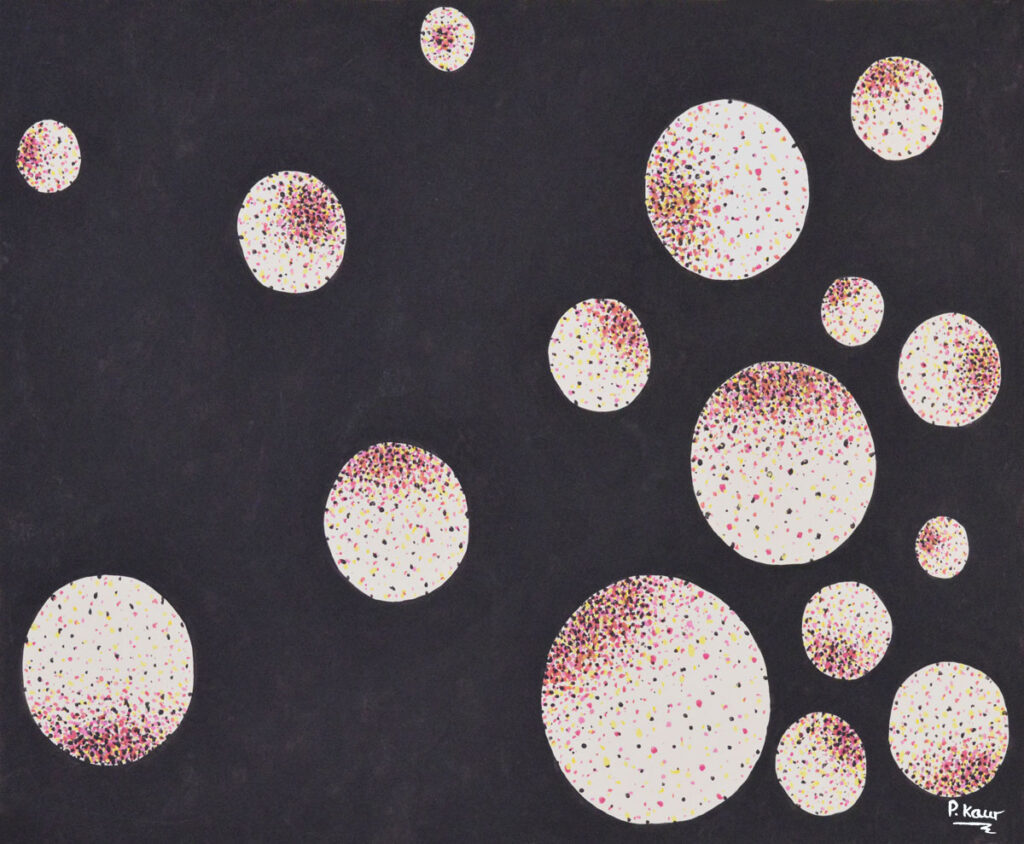
40 x 53cm
There are systems of energy within other systems. Each system therefore has its own entropy, like a battery. This theory is commonly applied to the cleanliness of a bedroom, stating that the natural state of a bedroom is one of chaos and disorder, where everything is the room is spread out evenly and everywhere rather than ordered and tidy.
Entropic Flower

40 x 53cm
This piece is a colourful rendition of entropy, demonstrating the petals as the energy that is being distributed to evenness and disorder.